“One little boy had no other choice. He was fitted with a traditional cast while in the care of his elderly and disabled grandparents and was unable to keep the cast dry in the shower. He continued wearing the wet cast for three weeks, inducing permanent scarring from skin breakdown. Even worse, his injury did not heal correctly and required another cast,” says Diana Hall, founder and CEO of ActivArmor.

ActivArmor offers a range of fully customizable casting for patient-specific needs. Photo credit: Aspose Pty Ltd.
“That’s when I knew. There was a solution in 3D printed plastics that could be customizable, fitted to any patient to accommodate native anatomy,” Hall says.

Founder and CEO, Diana Hall wearing an ActivArmor cast. Photo credit: Aspose Pty Ltd.
Additive manufacturing (AM) is not new to the medical field, but the 3D printing of immobilization devices is. ActivArmor, a startup company founded by Diana Hall in 2014, creates Class 1 devices for patients requiring orthoses — the correction of disorders of the limbs — by utilizing three-dimensional scanning to print customizable splints and casts for immobilization.
Problems That Plague Conventional Casts
Challenges with traditional casting can affect patients of any age but greatly impact children, specifically in terms of hygiene. Wear and tear is all too real in these instances and ultimately, can lead to further difficulties down the road to healing properly. As that little boy experienced, a conventional cast can trap moisture and bacteria, potentially leading to scarring. Thermal injuries and other complications are possible as well — incorrect placement, casting removal, repeat visits — all result from traditional casting methods. 3D printing can alleviate many of these challenges.
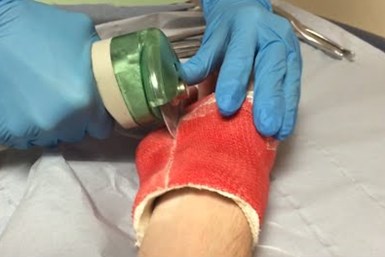
Oftentimes, removing traditional cast material proves time consuming and dangerous as a saw is used cut through the cast. Photo credit: ActivArmor
Hall, a chemical engineer with a background in software development, first made the connection between casts and 3D printing while running a mentoring program for children in poverty. She noticed these children, subject to substandard living conditions, often had injuries resulting in casts or splints.
Hall says the casts she noticed were made of fiberglass or plaster and would get filthy. The children wearing them were unable to practice basic hygiene, such as bathing or washing up for a snack. In a more extreme case, she recalls one little girl had bed bugs living under her cast because she had been sleeping on her grandparents’ floor.
“The kids didn’t have access to or knowledge of good hygiene practices; the casts got to the point where they would smell or be soggy,” Hall remembers.
“I couldn’t help but view the conventional [casting method] as 1800s technology.”
Applying Inspiration
The previously mentioned little boy who got his cast wet prompted Hall’s lightbulb moment. “I measured the little boy’s arm with calipers and 3D printed him a plastic cast with the same type of plastic Legos are made of,” says Hall, referring to the ABS still used for most ActivArmor devices.
Hall prompted the boy to check with his physician to see if he could effectively wear the 3D printed cast. “[The physician] asked me if I could make more,” she says. From this moment on, Hall worked to ensure her casting was both legal as well as successful in healing injuries, not only for children, but people of every demographic and lifestyle.
Varied Cast and Splint Options
“First, I called the FDA to see if I could legally do this. Could I really print casts if physicians wanted to order them?” Hall says.
At this point, around 2014, the FDA was still developing test protocols for 3D printed wearables. Hall offered her services as her background in chemical engineering enabled her to test for specifics such as microporosity and biocompatibility of 3D printed devices. Collaborating with the FDA, Hall was able to complete a large portion of required testing for ActivArmor for free.
After undergoing the required testing and careful inspection, Hall was able to register and list her wearables as Class 1 Devices. ActivArmor thus functions as a centralized manufacturing site selling nonsterile, extremity splints for immobilization.
The ActivArmor Process
The ActivArmor process involves multiple tools and technologies alongside the application of 3D printing to create fully customized casting.

Physician utilizes ActivArmor’s scanning app to ensure full customization of the limb. Photo credit: ActivArmor
To simplify, the physician marks up the affected limb with a marker, then inputs the measurements into ActivArmor Scan — a free smartphone app designed to scan and map the injured limb. The data and positioning measurements obtained from the scan are then imported into an in-house software system (ActivArmor Designware) to create a fully customizable digital casting design.
The software was created in collaboration with Oom Studios for design development along with Twikit to serve as the platform. Create-it-Real assisted ActivArmor with slicing integration.
This joint technology effort generates readily printable designs by exporting a code compatible with any 3D printer technology — to be printed by a fabrication partner or at ActivArmor’s facility using its Fusion 3 Edge printers.
A Customizable Printed Product
The application of 3D printing enables ActivArmor to produce customized casts based on individual patient need. The devices are most widely used in pediatrics and for pro athletes today but can treat a variety of conditions and types of patients.
“There's an infinite number of hands, wrists, and feet as well as body shapes and sizes. The design options physicians want depending on the patient’s injury are endless,” Hall says.
This is why ActivArmor can print a range of casting using multiple materials, including ABS plastic — a recyclable, thermoplastic polymer. Casts are printed in about two and a half hours using FFF (though Hall says other processes can be used as well). Typically, devices are ready to wear as printed, but additional finishing is also available when necessary.
“We do offer coatings used for increased strength as needed. For instance, fitting NFL players who take hard hits and need that extra strength and durability,” Hall shares.

A variety of ActivArmor casts and color options are available for a customized fit. Photo credit: ActivArmor
In addition to the varied color options for cast filament, there are limitless selections regarding texture and exterior. From thicker and more protective for added support in the case of more intense breaks, to thinner and more breathable for injuries requiring less support, there is an option for each patient’s need.
The flexibility in offering patients what they need not only in terms of support, but livability is the ActivArmor difference.

Wear and tear is not a confine of ActivArmor casting, as the material is waterproof as well as sanitizable. Photo credit: ActivArmor
Hall says the casting is designed to enable improved healing outcomes. ActivArmor’s attention to hygiene and livability translates directly into their orthoses capabilities: being water- and itch-proof, sanitizable, and easy to remove and adjust as needed. “ActivArmor has successfully fit over 3,000 patients with positive healing outcomes,” Hall says.
The Shift to Point-of-Care Printing
ActivArmor’s goal is to shift healthcare toward point-of-care 3D printing — doctors will have the ability to diagnose, scan and measure the affected limb, culminating in the ability to print casting in-clinic.
While most devices are made at the company’s facility in Colorado today, ActivArmor is launching a turnkey package that includes its proprietary software and a 3D printer for the fabrication of custom devices at point-of-care for quick-turn treatment. Training videos and supplemental content are offered online as well as on-site for physicians interested in obtaining the printing package.
There are monthly licensing packages available for any size clinic or hospital as ActivArmor is currently open to licensing its software, with the goal of obtaining about 20 licensures by the end of 2023.

An ActivArmor cast being printed at a fabrication site. Once the print is complete, the cast is ready for patient use. Photo Credit: ActivArmor
Hall says ActivArmor will dramatically change patient care in terms of treatment options, lending to decreased cost and increased livability as well as improving healing outcomes and hygienics for patients.
Furthermore, in-clinic printing reduces material waste — each cast uses only the necessary amount of plastic (no more, no less) and can be easily removed and replaced, eliminating the need for clinicians to make multiple casts.
Peek into ActivArmor’s AM Future (in AI)
Where conventional casting success depends on the knowledge and skill of individual technicians, ActivArmor’s digital process offers opportunities to capture and distribute information about device design that is fully connected to healing outcomes. One such advancement Hall mentions is the possibility of artificial intelligence (AI) and machine learning integration for casting in the future.
AI has the potential to promote patient care and treatment through data-driven results. The application can offer increased accuracy in measurements, leading to more comfortable as well as quicker casting and ultimately, a faster recovery time for patients.
Hall reveals ActivArmor plans to integrate AI functions in 2024 and is already collecting data on the possible implementation of AI in its process and goal to improve patient care. According to Hall, thousands of test scans have been completed in correlation with diagnoses.
This data is being collected to then feed and fuel algorithms supporting consistent treatment options for patients. AI would benefit the ActivArmor process in already existing templates and in simplifying the scanning process for doctors and patients alike.
“It takes some of the work out, and it also reduces human error, as there's a lot of training issues and turnover with casting tech staff, as well as orthopedic staff,” Hall says. “AI could give them a guideline to start with and would ultimately improve patient healing outcomes in the long run.”
Related Content
DMG MORI: Build Plate “Pucks” Cut Postprocessing Time by 80%
For spinal implants and other small 3D printed parts made through laser powder bed fusion, separate clampable units resting within the build plate provide for easy transfer to a CNC lathe.
Read MoreVulcanForms Is Forging a New Model for Large-Scale Production (and It's More Than 3D Printing)
The MIT spinout leverages proprietary high-power laser powder bed fusion alongside machining in the context of digitized, cost-effective and “maniacally focused” production.
Read MoreAdditive Manufacturing Is Subtractive, Too: How CNC Machining Integrates With AM (Includes Video)
For Keselowski Advanced Manufacturing, succeeding with laser powder bed fusion as a production process means developing a machine shop that is responsive to, and moves at the pacing of, metal 3D printing.
Read MoreAluminum Gets Its Own Additive Manufacturing Process
Alloy Enterprises’ selective diffusion bonding process is specifically designed for high throughput production of aluminum parts, enabling additive manufacturing to compete with casting.
Read MoreRead Next
3D Printing Startup to Deliver Thousands of Custom Hearing Aids Over Next Five Years
Starting with a pilot program in Jordan, nonprofit 3DP4ME is developing workflows to 3D print hearing aid earmolds and prosthetics near the people who need them.
Read MoreDental Lab Brings 3D Printing into Digital Dentistry Workflow
With 3D scan technology and resin-based 3D printers, Spectrum Dental Printing is changing the way dental devices are made — and potentially, how dentistry happens.
Read MoreHow A Digital Manufacturing Workflow Is Making Orthoses, Prostheses More Accessible
Digitization of the workflow needed to create limb prostheses, orthoses, helmets and more means that these devices will become more readily available. Using 3D scanning and 3D printing, EastPoint Prosthetics and Orthotics and Additive America have established a way to produce these devices that both changes lives and makes business sense.
Read More