Consortium Aims to Print COVID-19 Test Swabs at Rate of Millions Per Week
Nasopharyngeal swabs are now available for order and immediate fulfillment through members of this consortium, including: Carbon, Formlabs, Envisiontec and Origin.
A consortium spanning academia, medical and commercial enterprises to deliver clinically tested, FDA registered, 3D printed COVID-19 nasopharyngeal test swab designs at scale. Nasopharyngeal swabs are now available for order and immediate fulfillment through members of this consortium, including: Carbon, Formlabs, Envisiontec and Origin.
In an effort to relieve the bottleneck for coronavirus testing, this consortium of experts and thought leaders has developed and manufactured fully 3D printed, FDA registered test swabs with superior or equivalent efficacy to flocked swabs.
Validated manufacturers around the country are poised to rapidly produce up to 4 million 3D printed swabs per week. Hospital incident commanders can connect with members of this consortium directly and contact their state Emergency Management Agencies to request immediate supplies for their growing needs.
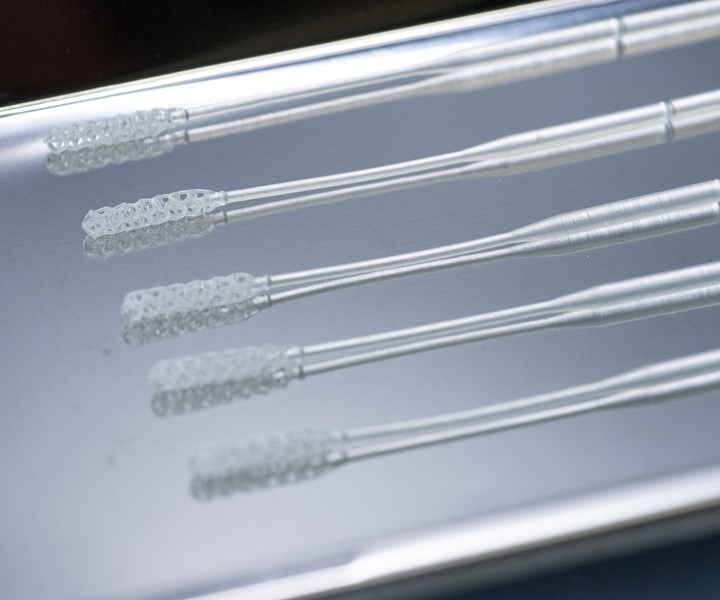
All manufacturers in this program have been verified as ISO13485 facilities and are in production on FDA registered swab designs.
All medical facilities are invited to complete a form for rapid response and fulfillment, the companies say.











