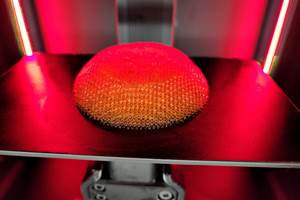
AddUp and Anatomic Implants Collaborate on FDA Submission for 3D Printed Toe Joint Replacement
Anatomic Implants has chosen to use AddUp’s FormUp 350 Powder Bed Fusion machine to qualify the implant for submission to the FDA. The FormUp 350 is able to produce varying complex geometries with fine detailed lattice structures, which are well suited for implantable medical devices.
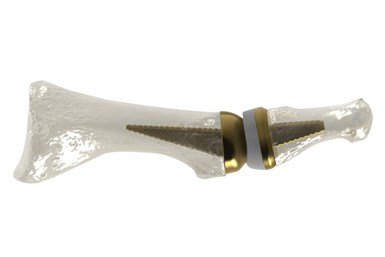
AddUp and Anatomic Implants, a medical device startup based in Washington, D.C., are working together on a 510(k) submission for what they say is the world’s first 3D printed toe joint replacement. Anatomic Implants says it is the first medical device startup company to patent and develop a 1st metatarsophalangeal (MTP) joint replacement that nearly perfectly replicates the human anatomy by leveraging titanium 3D printing technology.
The MTP toe joint is located at the base of the big toe and is one of the three main points used for balance. It is often the first joint in the foot to develop osteoarthritis. According to the company, the global market for the 1st MTP joint reconstruction is more than $500 million each year, while the market is underserved with very few products — none of which are anatomic or have the potential to support bone-in growth as well as the Anatomic Great Toe Joint.
Through the use of additive manufacturing (AM), a porous structure can be integrated into the design to promote osseointegration. Osseointegration gives implants a much higher chance of bonding to the bone, which can reduce the chances of the implant being rejected by the body. This can also lead to better patient outcomes after surgery.
To bring its product to market, the company has chosen AddUp’s FormUp 350 Powder Bed Fusion (PBF) machine to qualify the implant for submission to the FDA. The FormUp 350 is able to produce varying complex geometries with fine detailed lattice structures, which are well suited for implantable medical devices. The FDA’s Center for Devices and Radiological Health (CDRH) and the Center for Drug Evaluation and Research (CDER) has approved many 3D printed class II medical devices through the 510(k) pathway since the mid-2000s.
“With 1st MTP joint replacement being a largely underserved market, and medical device companies building lattice structures into implantables since the mid 2000s, Dr. Nutter and I sought out to make a more anatomic design by leveraging the latest technologies adopted by the industry and FDA,” says David Nutter, Anatomic Implants president. “We were excited to partner with AddUp to achieve 510(k) clearance after learning about their proprietary 3D printing technology and seeing how it could benefit the development of the Anatomic Great Toe Joint. We look forward to leveraging the AddUp team and their expertise to validate the world’s first 3D printed toe joint replacement on their FormUp 350.”
The 510(k) clearance process involves a comprehensive review of safety and performance data for the implant to determine if it is substantially equivalent to an implant that is already on the market. Several tests have already been completed and the company anticipates 510(k) clearance in late Q3 2024. Anatomic Implants has been working on the Anatomic Great Toe Joint since its inception late 2016 and has already secured design patents in the U.S., Canada and throughout Europe — which represents the majority of the global 1st MTP joint reconstruction market.
AddUp has a lot of experience in the medical industry with global OEMs relying on the FormUp 350 for serial production of their medical implants. The company’s North American subsidiary, The AddUp Solution Center, is located in Cincinnati, Ohio, and is ISO13485 certified. It has experience partnering with a variety of medical customers and supporting their path to FDA clearance.
“AddUp is committed to supporting the development of cutting-edge solutions for the medical market,” says Nick Estock, AddUp Deputy CEO. “Our team at the AddUp Solution Center has the expertise on FDA regulations and qualification protocols to provide a proactive approach to regulatory compliance essential for a successful 510(k) submission. We are excited to be supporting Anatomic Implants through this process to bring the first additively manufactured toe joint replacement to market.”
Anatomic Implants was founded by Johns Hopkins Surgeon Scott W. Nutter, DPM, and David Nutter. Both share a passion for innovation, entrepreneurship and helping people thrive through enabling them to walk better. Dr. Nutter served as chief of podiatry at Washington Adventist Hospital (now the White Oak Medical Center) for several years. He has performed many 1st MTP joint replacements, and has used every total joint replacement system that has been developed for the 1st MPJ. It was through his experience as a double board-certified physician that he had a desire to create a better product to support patient needs.
David Nutter is an entrepreneur with a track record of successful startups across a variety of industries. David contracted Jalex Medical, a medical device design and regulatory affairs firm, in 2017 to jumpstart the development of the Anatomic Great Toe Joint.
Related Content
Partners Improve Wheelchair Seats, Cushions Using 3D Printed Programmable Foam
The 3D printed programmable foam is said to enhance orthopedic seats and cushions, offering improved comfort and reliability for users.
Read MoreStratasys, CollPlant Unite Technologies for Industrial-Scale Bioprinting of Tissues, Organs
The joint development and commercialization agreement will initially focus on development of a bioprinting solution for CollPlant’s regenerative breast implants, addressing $2.6 billion market opportunity.
Read MoreNMPA Certifies Farsoon 3D Printed Tantalum Interspinal Fusion Cage
The company says the additively manufactured implants can be fully customized according to patients’ conditions, and the trabecular microstructure can achieve a high porosity of 68-78% to promote bone tissue and vessel fusion.
Read MoreMore Affordable Suture Anchors 3D Printed from PEKK: The Cool Parts Show #60
Selective laser sintering (SLS) of polyether ketone ketone (PEKK) is being used to produce medical implants that are more cost effective and perform better than their conventional counterparts. We highlight fasteners known as suture anchors in this episode of The Cool Parts Show.
Read MoreRead Next
Video: Intelligent Layering Metal 3D Printing at 3DEO
Contract manufacturer 3DEO delivers metal parts using Intelligent Layering, a binder jetting-like 3D printing process the company developed and operates internally. Here’s how it works.
Read MoreLooking to Secure the Supply Chain for Castings? Don't Overlook 3D Printed Sand Cores and Molds
Concerns about casting lead times and costs have many OEMs looking to 3D print parts directly in metal. But don’t overlook the advantages of 3D printed sand cores and molds applied for conventional metal casting, says Humtown leader.
Read MoreGE Additive Rebrands as Colibrium Additive
As part of the brand name transition, both the Concept Laser and Arcam EBM legacy brands will be retired.
Read More








.png;maxWidth=300;quality=90)